Hardenability is the property of steel that determines the depth and distribution of hardness induced by quenching from the austenitizing temperature. Hardenability should not be confused with hardness or with maximum hardness. Hardness is a measure of the ability of a metal to resist penetration as determined by any one of a number of standard tests (Brinell, Rockwell, Vickers, etc). The maximum attainable hardness of any steel depends solely on carbon content and is not significantly affected by alloy content. Maximum hardness is realized only when the cooling rate in quenching is rapid enough to ensure full transformation to martensite. The as-quenched surface hardness of a steel part is dependent on carbon content and cooling rate, but the depth to which a certain hardness level is maintained with given quenching conditions is a function of its hardenability. Hardenability is largely determined by the percentage of alloying elements in the steel. However, austenite grain size, time and temperature during austenitizing, and prior microstructure also significantly affect the hardness depth.
Steel’s versatility is due to its response to thermal treatment. Although most steel products are used in the as-rolled or un-heat-treated condition, thermal treatment greatly increases the number of properties that can be obtained, because at certain “critical temperatures” iron changes from one type of crystal structure to another. This structural change, known as an allotropic transformation, is spontaneous and reversible and can be made to occur by simply changing the temperature of the metal.
In steel, the transformation in crystal structure occurs over a range of temperatures, bounded by lower and upper critical points. When heated, most carbon and low–alloy steels have a critical temperature range between 1300 – 1600° F. Steel above this temperature, but below the melting range, has a crystalline structure known as austenite, in which the carbon and alloying elements are dissolved in a solid solution. Below this critical range, the crystal structure changes to a phase known as ferrite, which is capable of maintaining only a very small percentage of carbon in solid solution. The remaining carbon exists in the form of carbides, which are compounds of carbon and iron and certain of the other alloying elements. Depending primarily on cooling rate, the carbides may be present as thin plates alternating with the ferrite (pearlite), as spheroidal globular particles at ferrite grain boundaries or dispersed throughout the ferrite, or as a uniform distribution of extremely fine particles throughout a “ferrite-like” phase, which has an acicular (needlelike) appearance, named martensite. In some of the highly alloyed stainless steels, the addition of certain elements stabilizes the austenite structure so that it persists even at very low temperatures (austenitic grades). Other alloying elements can prevent the formation of austenite entirely up to the melting point (ferritic grades).
Fundamentally, all steel heat treatments are intended to either harden or soften the metal. They involve one or a series of operations in which the solid metal is heated and cooled under specified conditions to develop a required structure and properties.
The choice of quenching media is often a critical factor in the selection of steel of the proper hardenability for a particular application. Quenching severity can be varied by selection of quenching medium, agitation control, and additives that improve the cooling capability of the quenchant. Increasing the quenching severity permits the use of less expensive steels of lower hardenability. However, consideration must also be given to the amount of distortion that can be tolerated and the susceptibility to quench cracking. In general, the more severe the quenchant and the less symmetrical the part being quenched, the greater are the size and shape changes that result from quenching and the greater is the risk of quench cracking. Consequently, although water quenching is less costly than oil quenching, and water quenching steels are less expensive than those requiring oil quenching, it is important to know that the parts being hardened can withstand the resulting distortion and the possibility of cracking.
Oil, salt, and synthetic water-polymer quenchants are also used, but they often require steels of higher alloy content and hardenability. A general rule for the selection of steel and quenchant for a particular part is that the steel should have a hardenability not exceeding that required by the severity of the quenchant selected. The carbon content of the steel should also not exceed that required to meet specified hardness and strength, because quench cracking susceptibility increases with carbon content. The choice of quenching media is important in hardening, but another factor is agitation of the quenching bath. The more rapidly the bath is agitated, the more rapidly heat is removed from the steel and the more effective is the quench. Listed below are some terms commonly associated with the quenching process:
Direct Quenching:
Quenching carburized parts directly from the carburizing operation.
Fog Quenching: Quenching in a mist.
Hot Quenching: An imprecise term used to cover a variety of quenching procedures in which a quenching medium is maintained at a prescribed temperature above 160° F (71° C).
Interrupted Quenching: A quenching procedure in which the workpiece is removed from the first quench at a temperature substantially higher than that of the quenchant and is then subjected to a second quenching system having a different cooling rate than the first.
Selective Quenching: Quenching only certain portions of a workpiece.
Slack Quenching: The incomplete hardening of steel due to quenching from the austenitizing temperature at a rate slower than the critical cooling rate for the particular steel, resulting in the formation of one or more transformation products in addition to martensite.
Spray Quenching: Quenching in a spray of liquid.
Time Quenching: Interrupted quenching in which the duration of holding in the quenching medium is controlled.
Direct Hardening: Through hardening is applied to medium and high carbon parts that possess sufficient carbon content for hardening through the entire depth of the part. The parts are heated and quenched (cooled) to fix the structure of the part in a hardened state. The best recognized through hardened part in the world is a diamond!
Fog Quenching: Quenching in a mist.
Hot Quenching: An imprecise term used to cover a variety of quenching procedures in which a quenching medium is maintained at a prescribed temperature above 160° F (71° C).
Interrupted Quenching: A quenching procedure in which the workpiece is removed from the first quench at a temperature substantially higher than that of the quenchant and is then subjected to a second quenching system having a different cooling rate than the first.
Selective Quenching: Quenching only certain portions of a workpiece.
Slack Quenching: The incomplete hardening of steel due to quenching from the austenitizing temperature at a rate slower than the critical cooling rate for the particular steel, resulting in the formation of one or more transformation products in addition to martensite.
Spray Quenching: Quenching in a spray of liquid.
Time Quenching: Interrupted quenching in which the duration of holding in the quenching medium is controlled.
Direct Hardening: Through hardening is applied to medium and high carbon parts that possess sufficient carbon content for hardening through the entire depth of the part. The parts are heated and quenched (cooled) to fix the structure of the part in a hardened state. The best recognized through hardened part in the world is a diamond!
Typical Heat Treatments for SAE Carbon Steels (Direct Hardening)
SAE No.
| Normalize, Deg. F
| Anneal, Deg. F
| Harden, Deg. F
| Quench
| Temper, Deg. F
|
|---|---|---|---|---|---|
1025 & 1030
| —
| —
| 1575-1650
| A
| To Desired Hardness
|
1033 to 1035
| —
| —
| 1525-1575
| B
| |
1036
| 1600-1700
| —
| 1525-1575
| B
| |
—
| —
| 1525-1575
| B
| ||
1038 to 1040
| 1600-1700
| —
| 1525-1575
| B
| |
—
| —
| 1525-1575
| B
| ||
1041
| 1600-1700 and/or
| 1400-1500
| 1475-1550
| E
| |
1042 to 1050
| 1600-1700
| —
| 1475-1550
| B
| |
1052 & 1055
| 1550-1650 and/or
| 1400-1500
| 1475-1550
| E
| |
1060 to 1074
| 1550-1650 and/or
| 1400-1500
| 1475-1550
| E
| |
1078
| —
| 1400-1500
a | 1450-1500
| A
| |
1080 to 1090
| 1550-1650 and/or
| 1400-1500
a | 1450-1500
| E
b | |
1095
| —
| 1400-1500
a | 1450-1500
| F
| |
—
| 1400-1500
a | 1500-1600
| E
| ||
1132 & 1137
| 1600-1700 and/or
| 1400-1500
| 1525-1575
| B
| |
1138 & 1140
| —
| —
| 1500-1550
| B
| |
1600-1700
| —
| 1500-1550
| B
| ||
1141 & 1144
| —
| 1400-1500
| 1475-1550
| E
| |
1600-1700
| 1400-1500
| 1475-1550
| E
| ||
1145 to 1151
| —
| —
| 1475-1550
| B
| |
1600-1700
| 1475-1550
| —
| B
|
- Slow cooling produces a spheroidal structure in these high-carbon steels that is sometimes required for machining purposes.
- May be water- or brine-quenched by special techniques such as partial immersion or time quenched. Otherwise, they are subject to quench cracking.
Indirect Hardening:
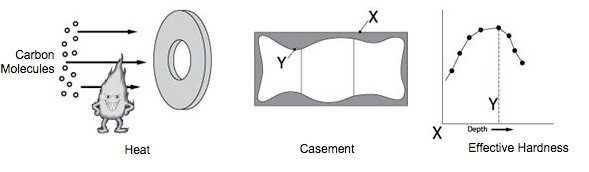
Case hardening (or indirect hardening) is applied to low-carbon content steel parts to increase surface hardness. During case hardening, carbon molecules are introduced to the part via solids, liquids, or gases in a process known as carburizing. The molecules penetrate the surface of the part, forming a casement, which is identified by the case depth (Y) and surface hardness (X). More exacting specifications will identify an effective case “Z” or a specific hardness requirement at a particular depth. Case hardness cannot be measured effectively using a Rockwell test. Readings must be taken from a cross section of the part using a microhardness tester.
Listed below are some terms and processes typically associated with case hardening (also known as indirect hardening):
Carburizing: A process in which carbon is introduced into a solid iron-base alloy by heating above the transformation temperature range while in contact with a carbonaceous material that may be a solid, liquid, or gas. Carburizing is frequently followed by quenching to produce a hardened case.
Case: 1) The surface layer of an iron-base alloy that has been suitably altered in composition and can be made substantially harder than the interior or core by a process of case hardening, and 2) the term case is also used to designate the hardened surface layer of a piece of steel that is large enough to have a distinctly softer core or center.
Carburizing: A process in which carbon is introduced into a solid iron-base alloy by heating above the transformation temperature range while in contact with a carbonaceous material that may be a solid, liquid, or gas. Carburizing is frequently followed by quenching to produce a hardened case.
Case: 1) The surface layer of an iron-base alloy that has been suitably altered in composition and can be made substantially harder than the interior or core by a process of case hardening, and 2) the term case is also used to designate the hardened surface layer of a piece of steel that is large enough to have a distinctly softer core or center.
Typical Heat Treatments for SAE Carbon Steels (Indirect hardening)
SAE No.
| Normalize, Deg. F
| Carburize, Deg. F
| Cool
a | Reheat Deg.F
| Cool
a | 2nd Reheat Deg. F
| Cool
a | Temper
b Deg. F
|
|---|---|---|---|---|---|---|---|---|
1010 to 1022
| —
| 1650-1700
| A
| —
| —
| —
| —
| 250-400
|
—
| 1650-1700
| B
| 1400-1450
| A
| —
| —
| 250-400
| |
—
| 1650-1700
| C
| 1400-1450
| A
| —
| —
| 250-400
| |
—
| 1650-1700
| C
| 1650-1700
| B
| 1400-1450
| A
| 250-400
| |
—
| 1500-1650
cd | B
| —
| —
| —
| —
| Optional
| |
1650-1750
f | 1350-1575
ed | D
| —
| —
| —
| —
| Optional
| |
1024
| 1650-1700
| E
| —
| —
| —
| —
| —
| 250-400
|
1025
1026 | —
| 1350-1575
ed | D
| —
| —
| —
| —
| Optional
|
—
| 1650-1700
| A
| —
| —
| —
| —
| 250-400
| |
1027
| —
| 1500-1650
cd | B
| —
| —
| —
| —
| Optional
|
1030
| —
| 1350-1575
ed | D
| —
| —
| —
| —
| Optional
|
—
| 1500-1650
cd | B
| —
| —
| —
| —
| Optional
| |
—
| 1350-1575
ed | D
| —
| —
| —
| —
| Optional
| |
1111
1112 1113 | —
| 1500-1650
cd | B
| —
| —
| —
| —
| Optional
|
—
| 1350-1575
ed | D
| —
| —
| —
| —
| Optional
| |
—
| 1650-1700
| A
| —
| —
| —
| —
| 250-400
| |
1109 to 1120
| —
| 1650-1700
| B
| 1400-1450
| A
| —
| —
| 250-400
|
—
| 1650-1700
| C
| 1400-1450
| A
| —
| —
| 250-400
| |
—
| 1650-1700
| C
| 1650-1700
| B
| 1400-1450
| A
| 250-400
| |
—
| 1500-1650
cd | B
| —
| —
| —
| —
| Optional
| |
—
| 1350-1575
ed | D
| —
| —
| —
| —
| Optional
| |
1126
| —
| 1500-1650
cd | B
| —
| —
| —
| —
| Optional
|
—
| 1500-1650
cd | B
| —
| —
| —
| —
| Optional
|
- Symbols: A = water or brine; B = water or oil; C = cool slowly; D = air or oil; E = oil; F = water, brine, or oil.
- Even where tempering temperatures are shown, tempering is not mandatory in many applications. Tempering is usually employed for partial stress relief and improves resistance to grinding cracks.
- Activated or cyanide baths.
- May be given refining heat as in other processes.
- Carbonitriding atmospheres
- Normalizing temperatures at least 50° F above the carburizing temperature are sometimes recommended where minimum heat-treatment distortion is of vital importance.
Thermal Modification of Steel
Listed below are terms and processes associated with the thermal modification of steel for compatibility with manufacturing. Manufacturing of steel frequently causes friction, which introduces heat to the material. Thermal modification of steel diminishes the potential for adverse consequences, such as deformation caused by heating.
Stress Relieving:
A process to reduce internal residual stresses in a metal object by heating the object to a suitable temperature and holding for a proper time at that temperature. This treatment may be applied to relieve stresses induced by casting, quenching, normalizing, machining, cold working, or welding.
Tempering:
Heating a quench-hardened or normalized ferrous alloy to a temperature below the transformation range to produce desired changes in properties.
Annealing:
Heating steel to and holding at a suitable temperature followed by cooling at a suitable rate, used primarily to soften but also to simultaneously produce desired changes in other properties or in microstructure. The purpose of the changes may be, but is not confined to, improvement of machinability, facilitation of cold working, improvement of mechanical or electrical properties, or increase in stability of dimensions. The time–temperature cycles used vary widely both in maximum temperature attained and in cooling rate employed, depending on the composition of the material, its condition, and the results desired.
Baking:
Heating to a low temperature in order to remove entrained gases.

